FE | Risk Minimisation Patient
Title
LNG-IUS is the most commonly used method by most HCPs involved in contraceptive counselling.5
HCP – healthcare professional; IUS – intrauterine system; LNG-IUS – levonorgestrel-releasing intrauterine system
- Addendum to the Clinical overview – Mirena® BAY86-5028 (Levonorgestrel intrauterine delivery system) No. 4.0. Date of report: APR 2021. Return to content
- Gemzell-Danielson K, Kubba A, Caetano C et al. Acta Obstet Gynecol Scand 2021. DOI: 10.1111/aogs.14110 [online ahead of print]. Return to content
- Gemzell-Danielson K, Kubba A, Caetano C et al. BMJ Sex Reprod Health 2021. DOI: 10.1136/bmjsrh-2020-200962 [online ahead of print]. Return to content
- PubMed. Search results for ‘Mirena’. Available at: https://pubmed.ncbi.nlm.nih.gov/?term=mirena [Accessed February 2021]. Return to content
- Gemzell-Danielson K, Cho S, Inki P et al. Contraception 2012;86(6):631–638. Return to content
Title
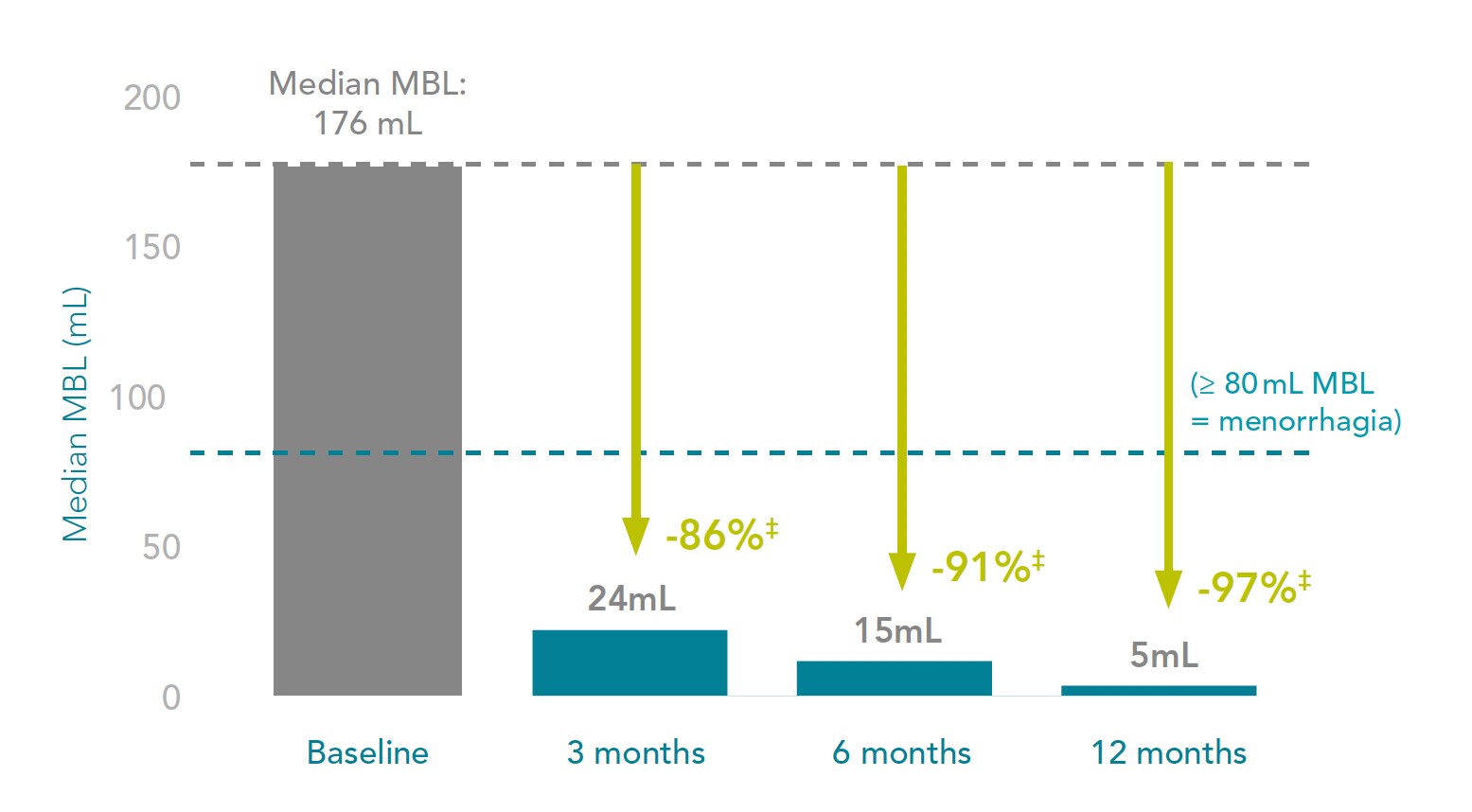
Mirena® reduces menstrual blood loss by 86%, as early as 3 months after placement1
MBL reduction in women with heavy menstrual bleeding after up to 12 months of Mirena® use*1
Adapted from Andersson JK and Rybo G. 1990.1
Recommend Mirena® as one of the most effective medical treatments for your patients with HMB1
HMB – heavy menstrual bleeding; MBL – menstrual blood loss
- Andersson JK and Rybo G. Obstet Gynaecol 1990;97(8):690–694. Return to content
Title
Mirena® targets the endometrium directly by locally releasing LNG1

Mirena®can be considered as a simple and effective alternative to surgical treatment in the management of HMB2
HMB – heavy menstrual bleeding; LNG – levonorgestrel
- Mirena® Summary of Product Characteristics. Return to content
- Naki MM, Tekcan C, Ozcan N et al. Fertil Steril 2010;94(1):371–374. Return to content
Title
Many patients seeking treatment for heavy menstrual bleeding may have questions regarding how therapy may impact their underlying fertility
There is no data from randomized controlled trials that has evaluated return to fertility in this specific population
It is not known if the return to fertility data from contraceptive studies is applicable to women seeking treatment for heavy menstrual bleeding2
- Mansour D. Contraception 2012;85(3):224–234. Return to content
- Mirena® Summary of Product Characteristics. Return to content
Title
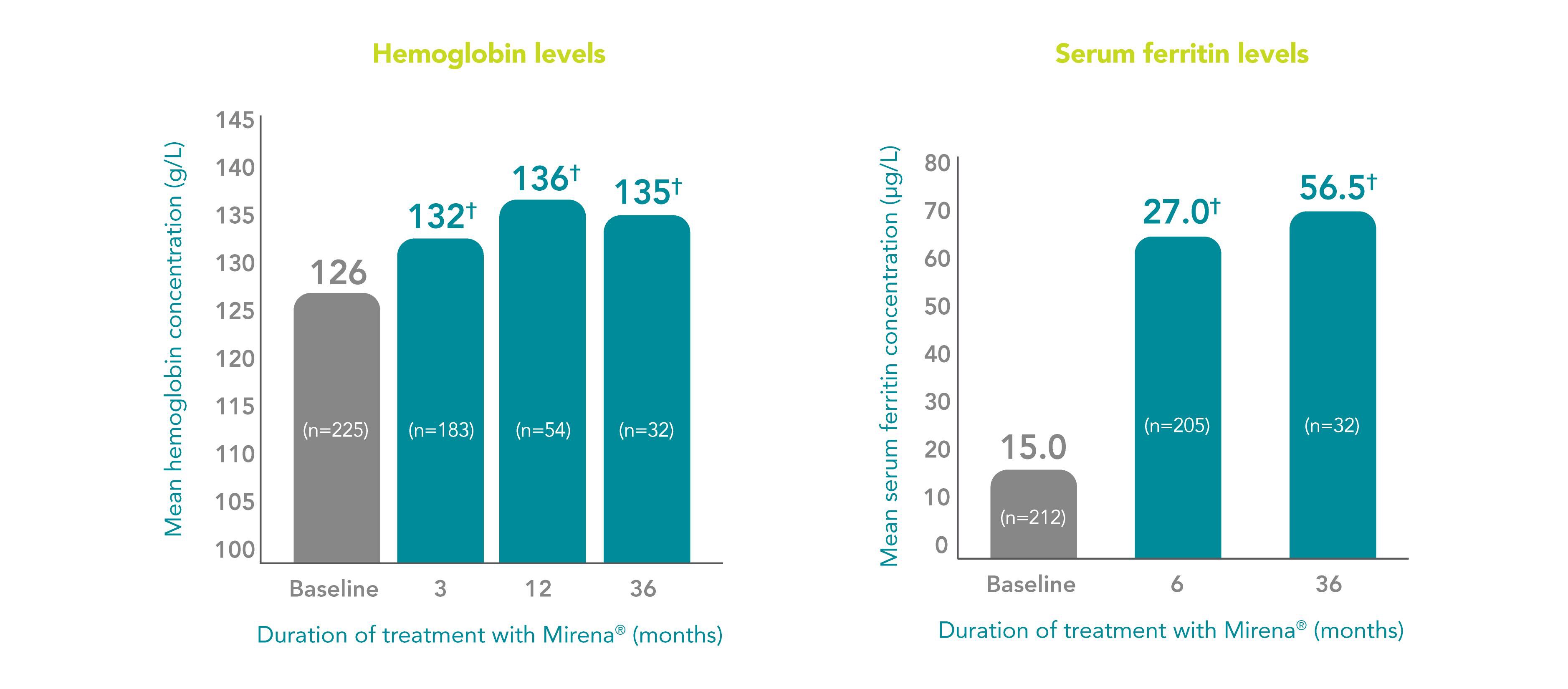
Mirena® improves hemoglobin and ferritin levels1
Median concentration of hemoglobin and serum ferritin in menorrhagic women at baseline and after up to 3 years of Mirena® use*1
Adapted from Endrikat J et al. 2012.1
Highlight the benefits of Mirena® to your patients with heavy menstrual bleeding-related anemia
- Endrikat J, Vilos G, Muysers C et al. Arch Gynecol Obstet 2012;285(1):117–121. Return to content
Title
Mirena®: for high satisfaction in women with heavy menstrual bleeding*1
|
|
Mirena® |
Endometrial |
|---|---|---|
|
“If I had a choice, I would |
100% |
56% |
|
“I feel much better |
100% |
72% |
|
“I noticed great improvements |
89% |
56% |
Consider Mirena® for significantly higher patient satisfaction vs endometrial ablation1
- Silva-Filho AL, Pereira FAN, Souza SS et al. Contraception 2013;87(4):409–415. Return to content
Title
Mirena® Internationally recognized as an effective treatment for women with heavy menstrual bleeding1-6
HMB – heavy menstrual bleeding
- NICE Clinical Guideline. Heavy Menstrual Bleeding: assessment and management. March 2018. Return to content
- ACOG. Obstet Gynecol 2013;122(1):176–185. Return to content
- Marret H, Fauconnier A, Chabbert-Buffet N et al. Eur J Obstet Gynecol Reprod Biol 2010;152(2):133–137. Return to content
- FSRH Clinical Guidance. Intrauterine Contraception. September 2019. Return to content
- FEBRASGO. Guia Prático de Condutas – Tratamento do Sangramento Uterino Anormal. 2014. Return to content
- Singh S, Best C, Dunn S et al. J Obstet Gynaecol Can 2013;35(5):473–475. Return to content
Title
Mirena®: Frequently asked questions
Below you will find answers to some commonly asked questions about the use of Mirena® for the treatment of heavy menstrual bleeding. These will support you when assessing appropriate treatment options for women in your clinical practice.
Mirena® is a long-acting reversible contraceptive method that can be particularly useful in women with heavy menstrual bleeding.1
- Mirena® Summary of Product Characteristics. Return to content
Mirena® is indicated for contraceptive use for up to 6 years and as a treatment option for menorrhagia for up to 5 years.1
If a woman wishes to continue using the same method, Mirena® can be replaced by a new system at the same time of the removal.1
- Mirena® Summary of Product Characteristics. Return to content
Undesirable effects are more common during the first months after the insertion of Mirena® and subside during prolonged use. Common side effects of Mirena® (occurring in less than 1 in 10 women), include:1
- Depressed mood/depression
- Nervousness
- Decreased libido
- Headache
- Dizziness
- Abdominal pain
- Nausea
- Acne
- Back pain
- Pelvic pain
- Dysmenorrhea
- Vaginal discharge
- Vulvovaginitis
- Breast tenderness
- Breast pain
- Intra-uterine contraceptive expelled
- Weight increase
Very common undesirable effects (occurring in more than 10% of users) include uterine/vaginal bleeding – spotting, oligomenorrhea, amenorrhea and benign cysts.1
For a full list of potential side effects, refer to the Summary of Product Characteristics. If a woman does experience side effects, she should speak to a doctor, pharmacist or nurse. This includes any possible side effects not listed in the contraception package leaflet.
- Mirena® Summary of Product Characteristics. Return to content
Weight gain has been reported as common in women fitted with Mirena® (occurring in more than 1 in 100 and less than 1 in 10 women). However, a causal link between Mirena® use and weight gain has not been established and requires further investigation.1
- Mirena® Summary of Product Characteristics. Return to content
Depression has been reported in fewer than 1 in 10 women fitted with Mirena®.1
There is no clear data to establish whether there is an increased risk of suicidal thoughts and behaviour associated with the use of hormonal contraceptives. Depressed mood and depression are well-known undesirable effects of hormonal contraceptive use. Depression can be serious and is a well-known risk factor for suicidal behaviour and suicide. Women should be advised to contact their doctor in case of mood changes and depressive symptoms, including shortly after initiating the treatment.2
- Mirena® Summary of Product Characteristics. Return to content
- European Medicines Agency. PRAC assessment on the use of combined hormonal contraception and suicidality [online]. Available at: https://www.ema.europa.eu/en/documents/prac-recommendation/prac-recommendations-signals-adopted-1-4-october-2018-prac-meeting_en.pdf [Last accessed February 2021]. Return to content
Mirena® is known to affect women’s menstrual cycles.1 Women fitted with Mirena® may experience lighter bleeding or some spotting (light bleeding in between periods) and irregular bleeding during the first 3–6 months after placement.2 Some women may have prolonged or heavy bleeding or an increase in the frequency of bleeding, usually in the first 2–3 months, before a reduction in blood loss is achieved.2 However, overall, women are likely to have fewer days of bleeding each month and may eventually have no periods at all. This is due to the effect of levonorgestrel on the lining of the womb.2
For women with menorrhagia, Mirena® usually results in lighter periods and can decrease blood loss by up to 90% at Cycle 3 and 96% at Cycle 6.1
- Reid PC and Virtanen-Kari S. BJOG 2005;112(8):1121–1125. Return to content
- Mirena® Summary of Product Characteristics. Return to content